Abstract
Introduction CD7-targeted chimeric antigen receptor T-cell (CAR-T) therapy is promising for T-cell malignancies. We have developed a novel fratricide-resistant approach to derive naturally selected 7CAR-T cells (NS7CAR) from bulk T-cells without additional genetic manipulations. Preliminary phase I trial results of 20 patients receiving NS7CAR were published in Blood. Here we reported the phase I/II clinical trials with longer observation and enrolled more patients in R/R T-ALL/LBL (NCT04572308 &NCT04916860).
Methods Peripheral blood (PB) mononuclear cells were collected from patients (n=51) or the transplant donor (n=2) by leukapheresis. T cells were purified using CD3 magnetic beads or, if needed, CD4 and CD8 magnetic beads. "Naturally selected" anti-CD7 CAR-T cells (NS7CAR) were transduced an anti-CD7 CAR into bulk peripheral T lymphocytes via a lentivirus vector. NS7CAR is a 2nd generation murine-based CAR-T containing 4-1BB and CD3ζ co-stimulatory domains. All patients received intravenous fludarabine (30 mg/m2/d) and cyclophosphamide (300 mg/m2/d) (FC) for 3 days before NS7CAR infusion. To control rapid disease progression, 48 patients received bridging chemotherapy.
Results From December 2020 to May 2022, 57 patients with R/R T-ALL/LBL were screened for this study. Due to rapid disease progression(n=1), production failure (n=1) or personal reason(n=2), 4 patients did not receive NS7CAR and were excluded from analysis.
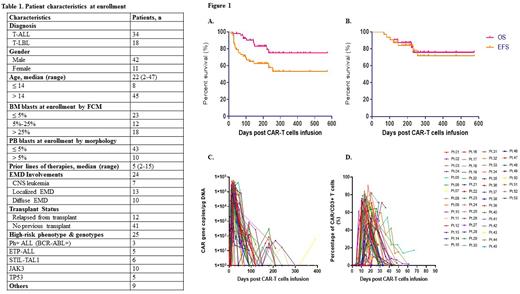
A total of 53 patients (T-ALL, n=34; T/LBL, n=18) received NS7CAR T-cell infusion. The median patient age was 22(2-47) years. Baseline patient characteristics are in Table 1. Three patients were Ph-positive T-ALL and 5 with early T-cell precursor ALL. Twenty-five patients had high-risk phenotypes and genotypes. At enrollment, 48 patients with bone marrow (BM) involvements, had a median percentage of BM blasts of 7.62 (0.05-87.72) % by flow cytometry (FCM), and 5 T-LBL patients without BM blasts. Ten patients had > 5% blasts in PB, with a median of 42% blasts (range: 6-62%).
Twenty-four patients had extramedullary disease (EMD), including central nervous system leukemia (n=8), diffuse involvements (n=10), and localized (n=13). Patients had a median of 5 (2-15) prior lines of therapy and 12 relapsed from prior transplant.
The transfection efficiency was 90% (40.7%-100%). A single dose of NS7CAR-T cells were infused at low-dose (5x105cells/kg, n=24), medium dose (1-1.5x106cells/kg, n=28) or high-dose (2x106cells/kg, n=1).
Up to the data cutoff July 15, 2022, the median follow-up time was 206 (45-574) days. On day 28, 95.8% (46/48) patients achieved minimal residual disease (MRD)-negative complete remission (CR) in BM/PB. Five T-LBL patients without BM blasts at enrollment remained MRD-negative. Twenty of 24 patients with EMD responded (13 CR and 7 PR) at a median of day 33 post infusion. For the 53 patients, the 18-month overall survival (OS) and event-free survival (EFS) was 75.0% and 53.1%, respectively (Fig.1A). Post infusion, 32 patients bridged to consolidation allo-HSCT within 3 months, and the 18-month OS and EFS was 75.8 and 71.5%, respectively (Fig.1B).
Among 21 patients without consolidation allo-HSCT, 11/21 less than CR underwent salvage transplantation. The other 7/21 patients remained in CR within a median of 88 (45-122) days. For the remaining 3 patients, 2 died within 5 months (1 PD, 1 relapse) and 1 lost to follow-up after 6 months.
In total, 8 relapsed, of 5 lost CD 7 expression. Four of 6 patients with STIL-TAL1 relapsed within 1-3months, and 3/4 lost CD7 expression.
Mild cytokine release syndrome (CRS, ≤grade II) occurred in 47/53 (88.7%) patients. Five patients had grade III CRS and 1 had grade IV CRS. Only 2 patients experienced grade I neurotoxicity.
The median peak of CAR-T copy number by qPCR was 1.75 ×105 (0.01-5.75 ×105) copies/µg DNA, which occurred on day 15(10-42) (Fig.1C). By FCM, NS7CAR proliferation reached a level as high as 90.52% of lymphocytes, with a median peak proportion of 63.95(0.54-90.52)% occurring on day 14 (7-32) (Fig.1D).
Conclusions Our phase I/II study shows that NS7CAR therapy is safe and highly effective in patients with heavily pretreated R/R T-ALL/LBL, including those with extramedullary involvements and a history of prior allo-HSCT. Patients with STIL-TAL1 tend to have a poorer response and early relapse. When relapse occurs, loss of CD7 expression was observed.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal